Merah Putih Vaccine set for Mass Production in 2022
Development progress of the Merah Putih vaccine has been encouraging. Amid the acceleration of vaccination, coordination should be intensified so we do not end up with more unused expired vaccines.
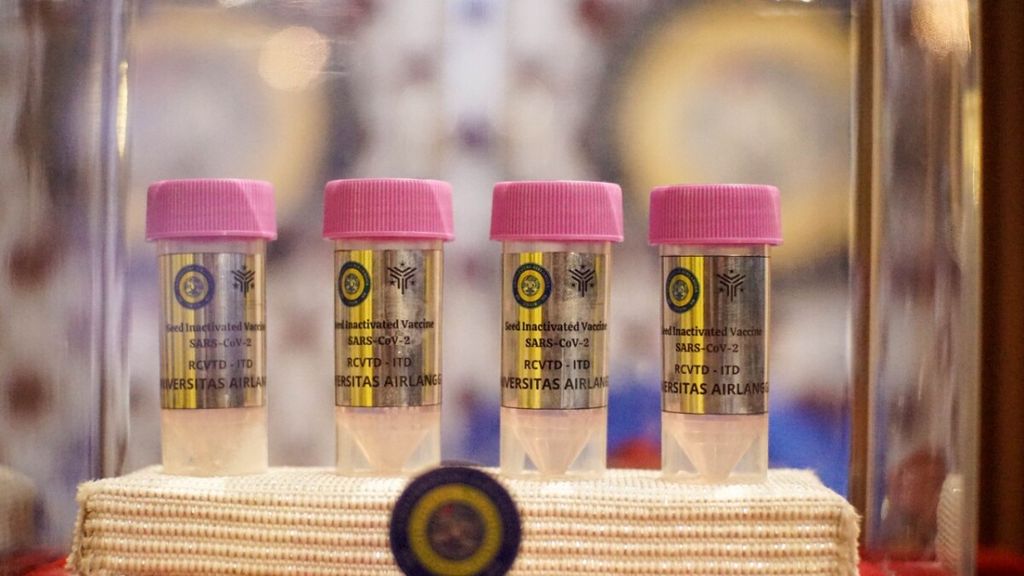
The Red and White Vaccine for handling Covid-19 from the Airlangga University research team is ready to be used for clinical trials. The vaccine seeds were given to PT Biotis Pharmaceuticals for clinical trial production. The delivery of vaccine seeds coincided with the Open Session of the 67th Anniversary of Airlangga University on Campus C Mulyorejo. Surabaya, East Java, Tuesday (9/11/2021).
JAKARTA, KOMPAS — The Merah Putih Covid-19 vaccine developed by Airlangga University, Surabaya, East Java, is ready for clinical trials. If everything runs smooth, the vaccine is expected to be mass produced in the second half of 2022.
The government has set a target that by the end of December, 50 percent of the total vaccination goal will have received a second dose. However, the management and coordination between different government levels need to be stepped up in order to prevent a recurrence of what happened in Kudus regency, Central Java, when 4,000 Covid-19 vaccine doses were wasted due to expiration.