AstraZeneca Vaccinations Halted Temporarily
Doses in this production group were distributed to the Indonesian Military (TNI) and partly to Jakarta and North Sulawesi.
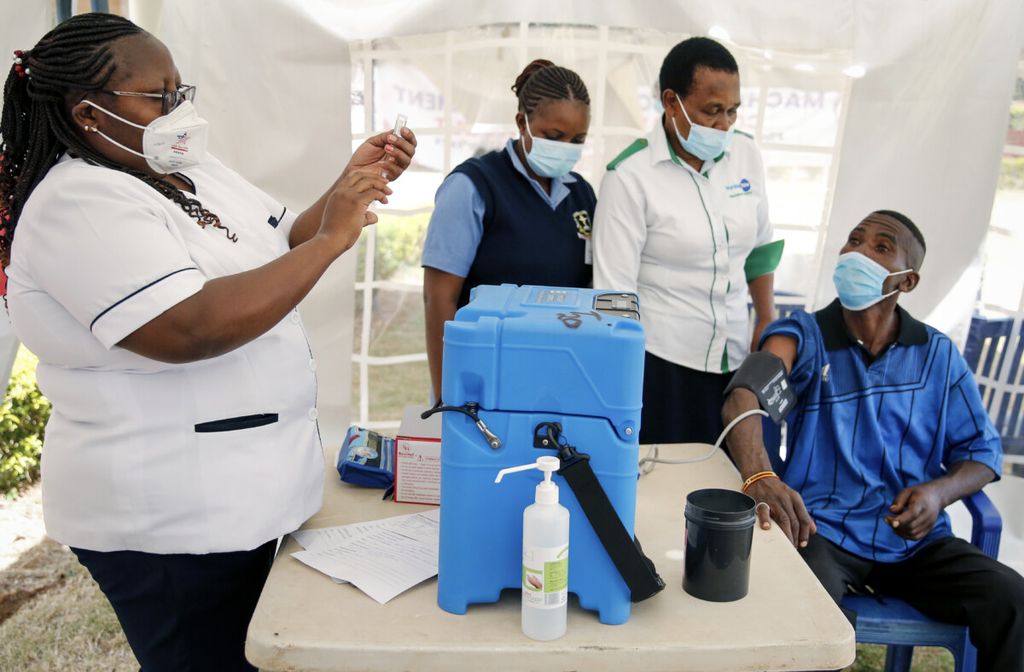
A nurse, left, prepares a shot of AstraZeneca COVID-19 vaccine, manufactured by the Serum Institute of India and provided through the global COVAX initiative, from a portable cold storage box, center, in Machakos, Kenya, Wednesday, March 24, 2021. AstraZeneca\'s repeated missteps in reporting vaccine data coupled with a blood clot scare could do lasting damage to the credibility of a shot that is the linchpin in the global strategy to stop the coronavirus pandemic, potentially even undermining vaccine confidence more broadly, experts say.
JAKARTA, KOMPAS — The government has temporarily halted the distribution and use of the CTMAV547 batch of COVID-19 vaccines made by AstraZeneca. The decision was made to improve testing at the Food and Drug Monitoring Agency (BPOM) and ensure the vaccine’s safety after reports of two fatal cases suspected of being related to AstraZeneca.
"This is a form of caution by the government to ensure the safety of this vaccine. The Health Ministry urges residents to be calm and not be consumed by hoaxes," Health Ministry’s vaccination spokesperson, Siti Nadia Tarmizi, said in a press statement in Jakarta on Sunday (16/5/2021).