Efficacy, Effectiveness and Immunogenicity
Various questions have emerged regarding the low efficacy of CoronaVac, the Covid-19 vaccine produced by Sinovac Biotech China, in Phase III of the clinical trials in Indonesia compared to in other countries.
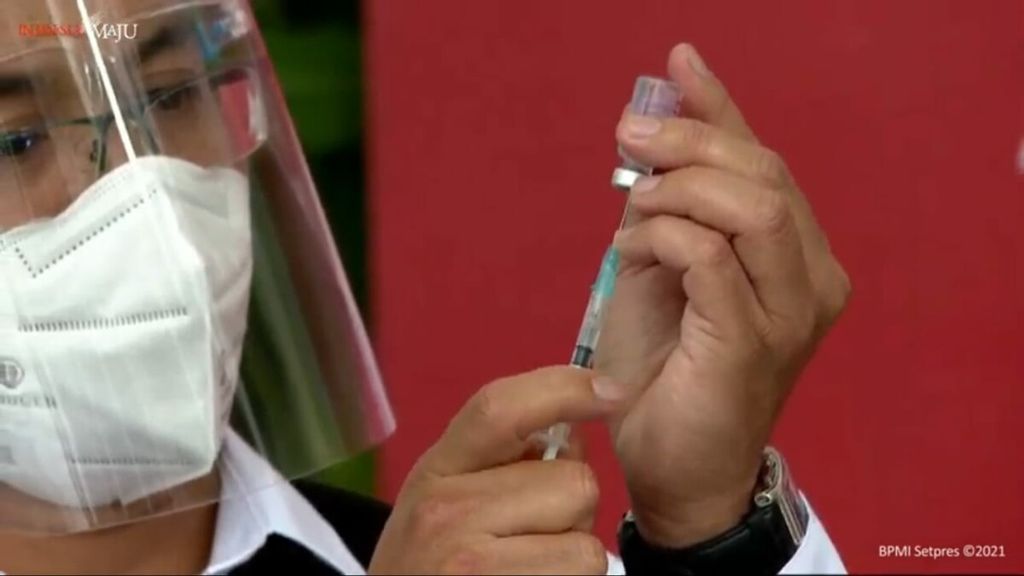
Medical officers are preparing to administer the CoronaVac vaccine, made by Sinovac Biotech Ltd, a company from China, Wednesday (13/1/2021) at Merdeka Palace, Jakarta. President Joko Widodo became the first person in Indonesia to receive a Covid-19 vaccine injection.
Various questions have emerged regarding the low efficacy of CoronaVac, the Covid-19 vaccine produced by Sinovac Biotech China, in Phase III of the clinical trials in Indonesia compared to in other countries. It was said that the efficacy of CoronaVac in Brazil was 78 percent, in Turkey 91.25 percent, while in Indonesia it was 65.3 percent with 99.23 percent immunogenicity up to three months after injection.
Efficacy, according to the website of the Global Alliance for Vaccines and Immunization (GAVI), is the extent to which the vaccine prevents disease, and if possible also prevents transmission, under ideal and controlled conditions. This is done by comparing the vaccinated group with the placebo group (receiving substances that have no therapeutic value). A vaccine with 65.3 percent efficacy in clinical trials meant a 65.3 percent reduction in cases of illness in the vaccinated group compared to other groups, which are neither vaccinated nor given placebo.